Jason D. Shields, Rachel Howells, Gillian Lamont, Yin Leilei, Andrew Madin, Christopher E. Reimann, Hadi Rezaei, Tristan Reuillon, Bryony Smith, Clare Thomson, Yuting Zhengc and Robert E. Ziegler (2024)
Highlighted by Jan Jensen
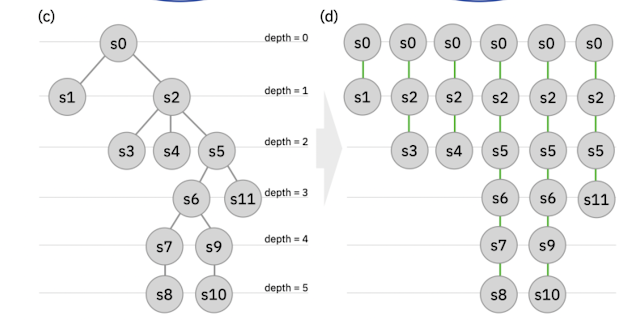
Figure 3 from this paper (c) the authors 2020. Reproduced under the CC-BY license
This is one of the rare papers where experimental chemists talk candidly about their experiences using ML models developed by others. In this case it is AiZynthFinder, which is developed at AstraZeneca Gothenburg and predicts retrosynthetic paths, while the users are most synthetic chemists at AstraZeneca in the UK, US, and China. The paper is really well written and well worth reading. I'll just include a few quotes below to whet your appetite.
"New users of AI tools in general are often disappointed by the failure of AI to live up to their expectations, and chemists' interaction with AiZynth is no exception. The first molecule that most new users test is one that they have personally synthesised recently, and AiZynthFinder rarely replicates their route exactly. Due in part to our self-imposed requirement to run fast searches, AiZynthFinder often gets close to a good route. Thus, experienced users seek inspiration from AiZynth rather than perfection."
"Common problems include proposals that would lead to undesired regioselectivity, functional group incompatibility, or overgeneralisation of precedented reactions to an inappropriate context."
"Early problems also included protection/deprotection cycles, which had to be intentionally penalised in order to focus AiZynth on productive chemistry. We have found that protecting group strategy is still best decided by the chemist. Thus, the AI proposals discussed in the case studies do not make heavy use of protecting groups, whereas several of the laboratory syntheses do."

This work is licensed under a Creative Commons Attribution 4.0 International License.