García-Rodeja, Y.; Solà, M.; Bickelhaupt , F. M.; Fernández, I. Chem. Eur. J. 2016, 22, 1368-1378
Contributed by Steven Bachrach
Reposted from Computational Organic Chemistry with permission
 '
'
This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach
Reposted from Computational Organic Chemistry with permission
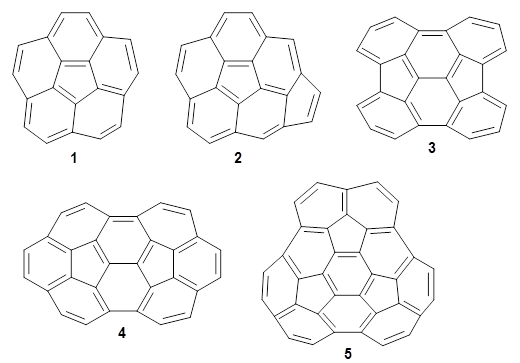
Fullerenes can undergo the Diels-Alder reaction with some specificity: the diene preferentially adds across the bond shared by two fused 6-member rings over the bond shared by the fused 6- and 5-member rings. Garcia-Rodeja and colleagues have examined the analogous Diels-Alder reaction of cyclopentadiene with five curved aromatic compounds, 1-5.1

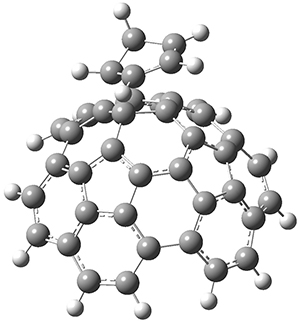
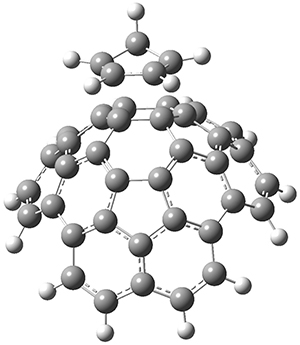
The computations were performed at BP86-D3/def2-TZVPP//RI-BP86-D3/def2-SVP. Representative transition states for the addition of cyclopentadiene with 3 over the 6,6-bond and 5,6-bond are shown in Figure 1.
5,6-bond
|
6,6-bond
|
Figure 1. RI-BP86-D3/def2-SVP optimized transition states for the reaction of cyclopentadiene with 3.
For the reactions of cyclopentadiene with 2-5 the reactions with the 6,6-bond is both kinetically and thermodynamically favored, while with 1 the 6,6-bond is kinetically preffered and the 5,6-adduct is the thermodynamic product. As the molecules increase in size (from 1 to 5), the activation barrier decreases, and the barrier for the reaction with 5 is only 1.4 kcal mol-1larger than the barrier with C60. The reaction energy also becomes more exothermic with increasing size. There is a very good linear relationship between activation barrier and reaction energy.
Use of the distortion/interaction model indicates that the preference for the 6,6-regioselectivity come from better interaction energy than for the 5,6-reaction, and this seems to come about by better orbital overlap between the cyclopentadiene HOMO and the 6,6-LUMO of the buckybowl.
References
(1) García-Rodeja, Y.; Solà, M.; Bickelhaupt , F. M.; Fernández, I. "Reactivity and Selectivity of Bowl-Shaped Polycyclic Aromatic Hydrocarbons: Relationship to C60," Chem. Eur. J. 2016, 22, 1368-1378, DOI:<10.1002/chem.201502248.
InChIs
1: InChI=1S/C20H10/c1-2-12-5-6-14-9-10-15-8-7-13-4-3-11(1)16-17(12)19(14)20(15)18(13)16/h1-10H
InChIKey=VXRUJZQPKRBJKH-UHFFFAOYSA-N
InChIKey=VXRUJZQPKRBJKH-UHFFFAOYSA-N
2: InChIKey=ASIFYFRJNYNQLA-UHFFFAOYSA-N
3: InChI=1S/C26H12/c1-5-13-14-6-2-11-19-20-12-4-8-16-15-7-3-10-18-17(9-1)21(13)25(22(14)19)26(23(15)18)24(16)20/h1-12H
InChIKey=OUWFOTSXASFGQD-UHFFFAOYSA-N
InChIKey=OUWFOTSXASFGQD-UHFFFAOYSA-N
4: InChI=1S/C30H12/c1-2-14-6-10-18-20-12-8-16-4-3-15-7-11-19-17-9-5-13(1)21-22(14)26(18)29(25(17)21)30-27(19)23(15)24(16)28(20)30/h1-12H
InChIKey=JEUCRZPADDQRKU-UHFFFAOYSA-N
InChIKey=JEUCRZPADDQRKU-UHFFFAOYSA-N
5: InChI=1S/C36H12/c1-7-16-17-9-3-14-5-11-20-21-12-6-15-4-10-19-18-8-2-13(1)22-25(16)31-32(26(18)22)34-28(19)24(15)30(21)36(34)35-29(20)23(14)27(17)33(31)35/h1-12H
InChIKey=QMGQDOOJOCPYIA-UHFFFAOYSA-N
InChIKey=QMGQDOOJOCPYIA-UHFFFAOYSA-N
 '
'This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment