Uchida, K.; Ito, S.; Nakano, M.; Abe, M.; Kubo, T. J. Am. Chem. Soc. 2016,138, 2399-2410
Contributed by Steven Bachrach
Reposted from Computational Organic Chemistry with permission
 '
'
This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Reposted from Computational Organic Chemistry with permission
Uchida and co-workers reported on the preparation of biphenalenylidene 1 and its interesting electrocyclization to dihydroperopyrene 2.1 The experimental barrier they find by experiment for the conversion of 1-Z to 1-E is only 4.3 kcal mol-1. Secondly, the photochemical electrocyclization of 2-antito 1-Z proceeds rapidly, through an (expected) allowed conrotatory pathway. However, the reverse reaction did not occur photochemically, but rather did occur thermally, even though this is formally forbidden by the Woodward-Hoffman rules.

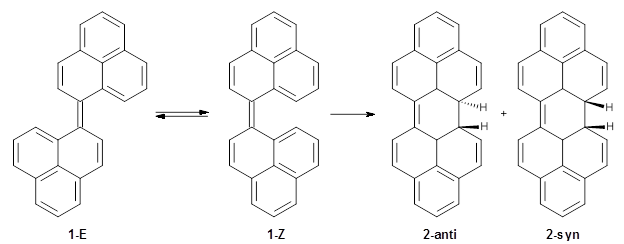
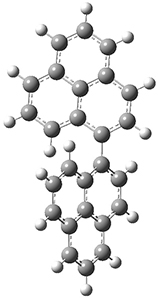
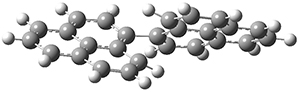
To address these issues, they performed a number of computations, with geometries optimized at UB3LYP(BS)/6-31G**. First, CASSCF computations indicated considerable singlet diradical character for 1-Z. Both 1-Z and 1-E show significant twisting about the central double bond, consistent with the inglet diradical character. 1-Z is 1.8 kcal mol-1 lower in energy than 1-E, and the barrier for rotation interconverting these isomers is computed to be 7.0 kcal mol-1, in reasonable agreement with the experiment. These geometries are shown in Figure 1.
1-Z
|
1-E
|
TS (Z→E)
| |
Figure 1. UB3LYP(BS)/6-31G** optimized geometries of 1-Z and 1- and the transition state to interconvert these two isomers.
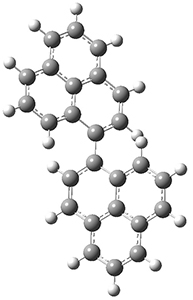
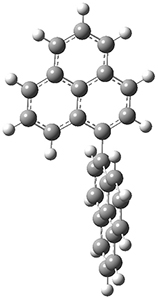
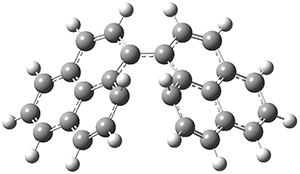
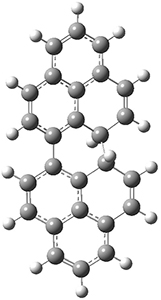
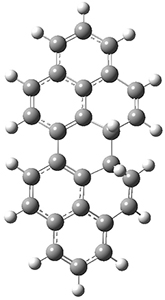
The conrotatory electrocyclization that takes 1-Z into 2-anti has a barrier of 26.0 kcal mol-1 and is exothermic by 3.4 kcal mol-1. The disrotatory process has a higher barrier (34.2 kcal mol-1) and is endothermic by 8.4 kcal mol-1. These transition states and products are shown in Figure 2. So, despite being orbital symmetry forbidden, the conrotatory path is preferred, and this agrees with their experiments.
TS (con)
|
TS (dis)
|
2-anti
|
2-syn
|
Figure 2. UB3LYP(BS)/6-31G** optimized geometries of 2-anti and 2-syn and the transition states leading to them.
The authors argue that the large diradical character of 1 leads to both its low Z→E rotational barrier, and the low barrer for electrocyclization. The Woodward-Hoffmann allowed disrotatory barrier is inhibited by its highly strained geometry, making the conrotatory path the favored route.
References
(1) Uchida, K.; Ito, S.; Nakano, M.; Abe, M.; Kubo, T. "Biphenalenylidene: Isolation and Characterization of the Reactive Intermediate on the Decomposition Pathway of Phenalenyl Radical," J. Am. Chem. Soc. 2016,138, 2399-2410, DOI: 10.1021/jacs.5b13033.
InChIs
1-E: InChI=1S/C26H16/c1-5-17-9-3-11-23-21(15-13-19(7-1)25(17)23)22-16-14-20-8-2-6-18-10-4-12-24(22)26(18)20/h1-16H/b22-21+
InChIKey=LOZZANITCNALJB-QURGRASLSA-N
InChIKey=LOZZANITCNALJB-QURGRASLSA-N
1-Z: InChI=1S/C26H16/c1-5-17-9-3-11-23-21(15-13-19(7-1)25(17)23)22-16-14-20-8-2-6-18-10-4-12-24(22)26(18)20/h1-16H/b22-21-
InChIKey=LOZZANITCNALJB-DQRAZIAOSA-N
InChIKey=LOZZANITCNALJB-DQRAZIAOSA-N
2-anti: InChI=1S/C26H18/c1-3-15-7-11-19-21-13-9-17-5-2-6-18-10-14-22(26(21)24(17)18)20-12-8-16(4-1)23(15)25(19)20/h1-14,19,21,25-26H/t19-,21-,25?,26?/m0/s1
InChIKey=BZIOOLOJBUBMSS-ATJINXRDSA-N
InChIKey=BZIOOLOJBUBMSS-ATJINXRDSA-N
2-syn: InChI=1S/C26H18/c1-3-15-7-11-19-21-13-9-17-5-2-6-18-10-14-22(26(21)24(17)18)20-12-8-16(4-1)23(15)25(19)20/h1-14,19,21,25-26H/t19-,21+,25?,26?
InChIKey=BZIOOLOJBUBMSS-YXGNQKCYSA-N
InChIKey=BZIOOLOJBUBMSS-YXGNQKCYSA-N
 '
'This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment