Melle-Franco, M. "Uthrene, a radically new molecule?," Chem. Commun. 2015, 51, 5387-5390
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Reposted from Computational Organic Chemistry with permission
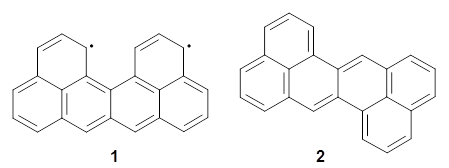
Uthrene 1 is the smallest formally diradical fragment of graphene; it cannot be expressed in a closed shell, fully electron-paired Kekule form. Its isomer zethrene 2 on the other hand, can be expressed in closed shell form.

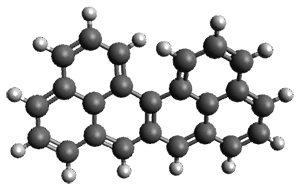
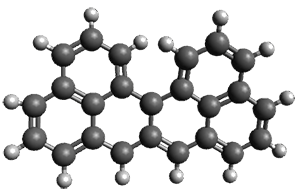
Melle-Franco has examined these, and related, polycyclic aromatic hydrocarbons with DFT.1 The optimized structure of singlet and triplet 1 at CAM-B3LYP/6-31g(d,p) are shown in Figure 1. The singlet-triplet energy gap of 2 is 16.5 kcal mol-1 with a ground state singlet. However, for 1 the triplet is predicted to be lower in energy than the singlet by 7.7 kcal mol-1. And this gap increases to 10.9 kcal mol-1 at CASSCF(14,14)/6-31g(d,p)//CAM-B3LYP/6-31g(d,p). Natural orbital population analysis of the singlet of 1 at CASSCF identifies two orbitals with populations around 1 e.
Interestingly, both the singlet and triplet of 1 are not planar, exhibiting a twist to avoid the clashing of the hydrogens in the bay region. (This twisting is best seen by clicking on the structures in Figure 1, and viewing the molecules interactively through Jmol.)
singlet
|
triplet
|
Figure 1. CAM-B3LYP/6-31g(d,p) optimized geometries of the singlet and triplet of 1.
References
(1) Melle-Franco, M. "Uthrene, a radically new molecule?," Chem. Commun. 2015, 51, 5387-5390, DOI:10.1039/C5CC01276G.
InChIs
1: InChI=1S/C24H14/c1-5-15-7-3-11-20-22(15)17(9-1)13-19-14-18-10-2-6-16-8-4-12-21(23(16)18)24(19)20/h1-14H
InChIKey=WAKUAPLTIMTENF-UHFFFAOYSA-N
InChIKey=WAKUAPLTIMTENF-UHFFFAOYSA-N
2: InChI=1S/C24H14/c1-5-15-7-3-11-19-22-14-18-10-2-6-16-8-4-12-20(24(16)18)21(22)13-17(9-1)23(15)19/h1-14H
InChIKey=UXUXNGMSDNTZEC-UHFFFAOYSA-N
InChIKey=UXUXNGMSDNTZEC-UHFFFAOYSA-N

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment