Chu, M.; Scioneaux, A. N.; Hartley, C. S. J. Org. Chem. 2014, 79, 9009–9017
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
π-π-stacking has been a major theme of my blog, and is discussed in Chapter 3.5.4 in the Second Edition of my book. Most examples involved molecules that are nearly circular (like benzene or triphenylene). Hartley and co-workers discuss the π-π-stacking of the oblong molecule 1, comparing its experimental features with computed features of the model compound 2.1

The key spectroscopic feature associated with assembly of 1 are the changes in the 1H chemical shifts with increasing concentration. For example, the chemical shift of the three protons on the triphenylene unit shift upfield by 0.30 to 0.66 ppm as the concentration increases from 10-5 to 10-2 M.
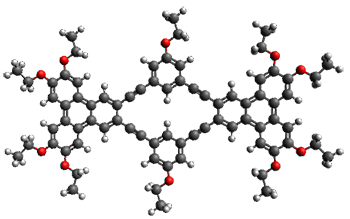
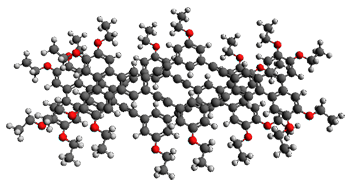
To see if these NMR shift changes are due to association of 1, they employed a computational approach. First they optimized the structure of model compound 2 at B3LYP/6-31G(d) (Shown in Figure 1a). Then using this fixed geometry, they computed the 1H chemical shifts of the dimer of 2. They explored the stacking distance (ranging from 3.2 to 4.0 Å along with varying the displacement of the two molecules along the major axis from 0.0 to 6.0 Å, finding the best fit to the chemical shifts with a separation of 3.6 Å and a displacement along the major axis of 3.5 Å. Using these two fixed values, they explored displacement of the molecules along the minor axis, along with rotation of the two molecules. The best fit to the experimental chemical shifts was with a displacement of 0.5 Å along the short axis and no rotation. This structure is shown in Figure 1b, with a RMS error of only 0.09 ppm from experiment. Models of the trimer show poorer fit to the experimental data.
(a)
2
|
(b)
2
dimer |
Figure 1. B3LYP/6-31G(d) (a) optimized structure of 2 and the (b) structure of the best fit of the dimer of2. (As always, clicking on these images will allow you to manipulate the 3-D structure using JMol – highly recommended for the dimer.)
Using some smaller models and the B97-D functional, they argue that the displacement, which is substantially larger than the displacement found in stacked triphenylene, results from the need to minimize the steric interactions between the alkoxyl chains.
References
(1) Chu, M.; Scioneaux, A. N.; Hartley, C. S. "Solution-Phase Dimerization of an Oblong Shape-Persistent Macrocycle," J. Org. Chem. 2014, 79, 9009–9017; DOI: 10.1021/jo501260c.
InChIs:
1: InChI=1S/C116H148O10/c1-11-21-31-41-59-117-95-71-87-51-55-91-75-97-99(103-81-111(121-63-45-35-25-15-5)115(125-67-49-39-29-19-9)85-107(103)105-83-113(123-65-47-37-27-17-7)109(79-101(97)105)119-61-43-33-23-13-3)77-93(91)57-53-89-70-90(74-96(73-89)118-60-42-32-22-12-2)54-58-94-78-100-98(76-92(94)56-52-88(69-87)72-95)102-80-110(120-62-44-34-24-14-4)114(124-66-48-38-28-18-8)84-106(102)108-86-116(126-68-50-40-30-20-10)112(82-104(100)108)122-64-46-36-26-16-6/h69-86H,11-50,59-68H2,1-10H3
InChIKey=UUKOQEVXPHHWBJ-UHFFFAOYSA-N
InChIKey=UUKOQEVXPHHWBJ-UHFFFAOYSA-N
2: InChI=1S/C76H68O10/c1-11-77-55-31-47-21-25-51-35-57-59(63-41-71(81-15-5)75(85-19-9)45-67(63)65-43-73(83-17-7)69(79-13-3)39-61(57)65)37-53(51)27-23-49-30-50(34-56(33-49)78-12-2)24-28-54-38-60-58(36-52(54)26-22-48(29-47)32-55)62-40-70(80-14-4)74(84-18-8)44-66(62)68-46-76(86-20-10)72(82-16-6)42-64(60)68/h29-46H,11-20H2,1-10H3
InChIKey=NGTKTKCZPONPTB-UHFFFAOYSA-N
InChIKey=NGTKTKCZPONPTB-UHFFFAOYSA-N

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment