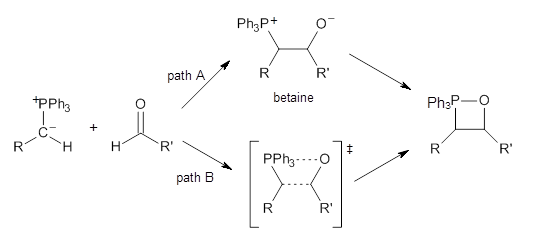
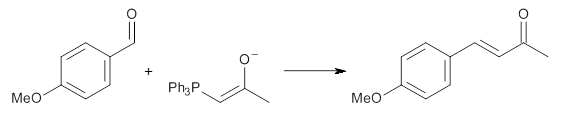
Contributed by +Jan Jensen
'
DOI: 10.1038/srep07022 CC-BY-NC-ND
Computational chemistry is finally in a position to conduct studies on hundreds or even thousands of molecules within a reasonable amount of time. This has led to benchmark datasets which have been used to develop new semi-empirical methods (e.g. DFT-Dx or HF-3c) as well as high-throughput screening of molecules with desirable properties.
This paper represents a third kind of study: a concerted effort to fill in missing experimental data needed to quantitatively understand a complex chemical system - in this case cellular metabolism. Standard Gibbs reaction free energies of biochemical reactions are crucial to quantitative modeling of metabolism (metabolic flux analysis) but only a small fraction have been measured experimentally.
Many of the reactions involve molecule-sizes that can be modeled routinely by electronic structure calculations. However, there are new challenges compared to, for example, atmospheric chemistry such as the modeling of pH- and solvation-effects and multiply charged ions. Thus, any conformational search must often be repeated for several different protonation states and multiply charged ions often necessitate the treatment of explicit solvent molecules and, even then, result in relatively solvation energies with relatively large errors. Also, multiply charged anions may present additional problems for DFT due to incomplete cancellation of the inter-electronic Coulomb repulsion at larger distances.
This paper represents the first, pioneering, attempt to address these issues with high-throughput in mind and the results are encouraging. For reactions involving non-multiply charged species B3LYP/6-31G* with the COSMO solvation model augmented by explicit water molecules result in MADs less than 5 kcal/mol, while being fast enough to compute more than a hundred reaction energies (involving more than 5000 quantum calculations).
However, this paper is more a call to action than the definitive answer. Many new methodological improvements (perhaps such as this one?) are needed in order to increase the accuracy and efficiency needed produce a complete and accurate picture of cellular metabolism. I hope this paper will spur such developments - hopefully in the form of large and open collaborations (perhaps similar to this one?).
Thanks to Adrian Jinich for alerting me to this paper

This work is licensed under a Creative Commons Attribution 4.0 International License.