Barua, S. R.; Quanz, H.; Olbrich, M.; Schreiner, P. R.; Trauner, D.; Allen, W. D. Chem. Eur. J. 2014, 20, 1638-1645
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
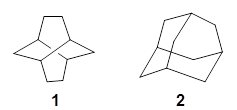
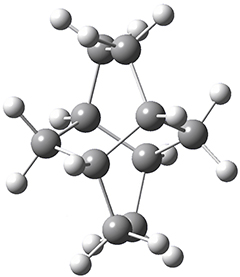
Twistane 1 is a more strained isomer of adamantane 2. The structure of 1 is shown in Figure 1.

1
|
Figure 1. B3LYP/6-31G(d) optimized structure of 1.
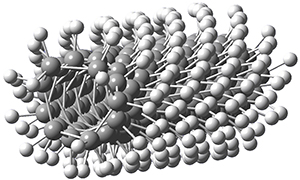
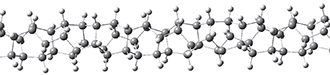
Adamantane is the core structure of diamond, which can be made by appending isobutene groups onto adamantane. In an analogous fashion, twistane can be extended in a linear way by appending ethano groups in a 1,4-bridge. Allen, Schreiner, Trauner and co-workers have examined this “polytwistane” using computational techniques.1 They examined a (CH)236 core fragment of polytwistane, with the dangling valences at the edges filled by appending hydrogens, giving a C236H242 compound. This compound was optimized at B3LYP/6-31G(d) and shown in Figure 2a. (Note that I have zoomed in on the structure, but by activating Jmol – click on the figure – you can view the entire compound.) A fascinating feature of polytwistane is its helical structure, which can be readily seen in Figure 2b. A view down the length of this compound, Figure 2c, displays the opening of this helical cylinder; this is a carbon nanotube with an inner diameter of 2.6 Å.
(a)
|
 (b) |
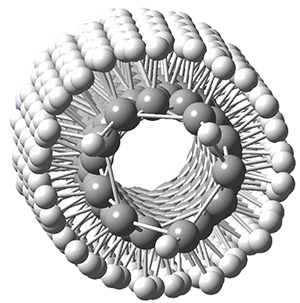 (c) |
Figure 2. B3LYP/6-31G(d) structure of the C236H242 twistane. (a) A zoomed in look at the structure. This structure links to the Jmol applet allowing interactive viewing of the molecule – you should try this! (b) a side view clearly showing its helical nature. (c) A view down the twistane showing the nanotube structure.
Though the molecule looks quite symmetric, each carbon is involved in three C-C bonds, and each is of slightly different length. The authors go through considerable detail about addressing the symmetry and proper helical coordinates of polytwistane. They also estimate a strain energy of about 1.6 kcal mol-1 per CH unit. This modest strain, they believe, suggests that polytwistanes might be reasonable synthetic targets.
References
(1) Barua, S. R.; Quanz, H.; Olbrich, M.; Schreiner, P. R.; Trauner, D.; Allen, W. D. "Polytwistane," Chem. Eur. J. 2014, 20, 1638-1645, DOI: 10.1002/chem.201303081.
InChIs
1: InChI=1S/C10H16/c1-2-8-6-9-3-4-10(8)5-7(1)9/h7-10H,1-6H2
InChIKey=AEVSQVUUXPSWPL-UHFFFAOYSA-N
InChIKey=AEVSQVUUXPSWPL-UHFFFAOYSA-N
2: InChI=1S/C10H16/c1-7-2-9-4-8(1)5-10(3-7)6-9/h7-10H,1-6H2
InChIKey=ORILYTVJVMAKLC-UHFFFAOYSA-N
InChIKey=ORILYTVJVMAKLC-UHFFFAOYSA-N

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment