Jašík, J.; Gerlich, D.; Roithová, J. J. Am. Chem. Soc. 2014, 136, 2960
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
Benzene is certainly one of the most iconic chemical compounds – its planar hexagonal structure is represented often in popular images involving chemists, and its alternating single and double bonds the source of one of chemistry’s most mythic stories: Kekule’s dream of a snake biting its own tail. So while the structure of benzene is well-worn territory, what of the structure of the benzene dication? Jasik, Gerlich and Rithova probe that question using a combined experimental and computational approach.1
The experiment involves generation of the benzene dication at low temperature and complexed
to helium. Then, using infrared predissociation spectroscopy (IRPD), they obtained a spectrum that suggested two different structures.
to helium. Then, using infrared predissociation spectroscopy (IRPD), they obtained a spectrum that suggested two different structures.
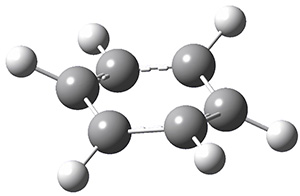
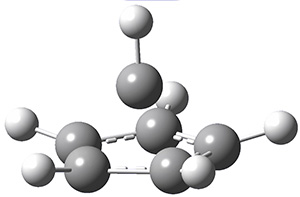
Next, employing MP2/aug-cc-pVTZ computations, they identified a number of possible geometries, and the two lowest energy singlet dications have the geometries shown in Figure 1. The first structure (1) has a six member ring, but the molecule is no longer planar. Lying a bit lower in energy is 2, having a pentagonal pyramid form. The combination of the computed IR spectra of each of these two structures matches up extremely well with the experimental spectrum.
1
|
2
|
Figure 1. MP2/aug-cc-pVTZ geometries of benzene dication 1 and 2.
References
(1) Jašík, J.; Gerlich, D.; Roithová, J. "Probing Isomers of the Benzene Dication in a Low-Temperature Trap," J. Am. Chem. Soc. 2014, 136, 2960-2962, DOI: 10.1021/ja412109h.

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment