Wagner, J. P.; Schreiner, P. R. J. Chem. Theor. Comput. 2014,10, 1353-1358
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
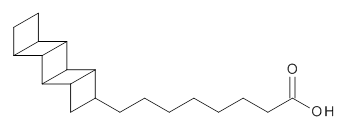
1

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
Schreiner provides another beautiful example of the important role that dispersion plays, this time in a biological system.1 The microbe Candidatus Brocadia Anammoxidans oxidizes ammonia with nitrite. This unusual process must be done anaerobically and without allowing toxic side products, like hydrazine to migrate into the cellular environment. So this cell has a very dense membrane surrounding the enzymes that perform the oxidation. This dense membrane is home to some very unusual lipids, such as 1. These lipids contain the ladderane core, a highly strained unit. Schreiner hypothesized that these ladderane groups might pack very well and very tightly due to dispersion.

1
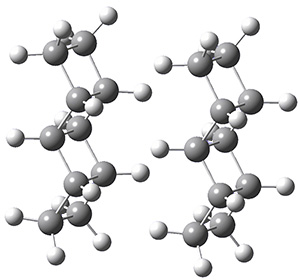
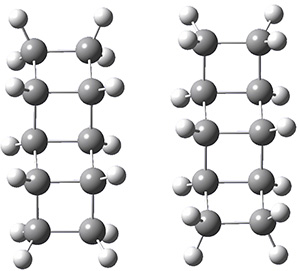
The geometries of the [2]- through [5]-ladderanes and their dimers were optimized at MP2/aug-cc-pVDZ, and the binding energies corrected for larger basis sets and higher correlation effects. The dimers were oriented in their face-to-face orientation (parallel-displaced dimer, PDD) or edge-to-edge (side-on dimer, SD). Figure 1 shows the optimized structures of the two dimeric forms of [4]-ladderane.
[4]-PDD
|
[4]-SD
|
Figure 1. MP2/aug-cc-pVDZ optimized geometries of the dimers of [4]-ladderane in the PDD and SD orientations.
The binding energies of the ladderane dimers, using the extrapolated energies and at B3LYP-D3/6-311+G(d,p), are listed in Table 1. (The performance of the B3LYP-D3 functional is excellent, by the way.) The binding is quite appreciable, greater than 6 kcal mol-1 for both the [4]- and [5]-ladderanes. Interestingly, these binding energies far exceed the binding energies of similarly long alkanes. So, very long alkyl lipid chains would be needed to duplicate the strong binding. Nature appears to have devised a rather remarkable solution to its cellular isolation problem!
Eextrapolated
|
EB3LYP-D3
| |
[2]-PDD
|
-27.
|
-3.2
|
[3]-PDD
|
-4.2
| -4.1 |
[4]-PDD
|
-5.5
|
-5.3
|
[5]-PDD
|
-6.6
|
-6.5
|
[2]-SD
|
-3.3
|
-3.1
|
[3}-SD
|
-4.1
|
-4.4
|
[4]-SD
|
-6.5
|
-5.7
|
[5]-SD
|
-7.5
|
-7.0
|
References
(1) Wagner, J. P.; Schreiner, P. R. "Nature Utilizes Unusual High London Dispersion
Interactions for Compact Membranes Composed of Molecular Ladders," J. Chem. Theor. Comput. 2014,10, 1353-1358, DOI: 10.1021/ct5000499.
Interactions for Compact Membranes Composed of Molecular Ladders," J. Chem. Theor. Comput. 2014,10, 1353-1358, DOI: 10.1021/ct5000499.
InChIs
1: InChI=InChI=1S/C20H30O2/c21-15(22)7-5-3-1-2-4-6-11-10-14-16(11)20-18-13-9-8-12(13)17(18)19(14)20/h11-14,16-20H,1-10H2,(H,21,22)/t11-,12-,13+,14-,16+,17+,18-,19-,20+/m0/s1
InChIKey=ZKKJRZDMLYQUNK-QIPWGTBCSA-N
InChIKey=ZKKJRZDMLYQUNK-QIPWGTBCSA-N
[2]-ladderane: InChI=1S/C6H10/c1-2-6-4-3-5(1)6/h5-6H,1-4H2/t5-,6+
InChIKey=YZLCEXRVQZNGEK-OLQVQODUSA-N
InChIKey=YZLCEXRVQZNGEK-OLQVQODUSA-N
[3]-ladderane: InChI=1S/C8H12/c1-2-6-5(1)7-3-4-8(6)7/h5-8H,1-4H2/t5-,6+,7+,8-
InChIKey=YTZCZYFFHKYOBJ-SOSBWXJGSA-N
InChIKey=YTZCZYFFHKYOBJ-SOSBWXJGSA-N
[4]-ladderane: InChI=1S/C10H14/c1-2-6-5(1)9-7-3-4-8(7)10(6)9/h5-10H,1-4H2/t5-,6+,7+,8-,9-,10+
InChIKey=VZHFDSXKIJOCAY-UXAOAXNSSA-N
InChIKey=VZHFDSXKIJOCAY-UXAOAXNSSA-N
[5]-ladderane: InChI=1S/C12H16/c1-2-6-5(1)9-10(6)12-8-4-3-7(8)11(9)12/h5-12H,1-4H2/t5-,6+,7-,8+,9+,10-,11-,12+
InChIKey=CWUAAECPDWVJSU-SBBGGFAWSA-N
InChIKey=CWUAAECPDWVJSU-SBBGGFAWSA-N

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment