M.-G. Song and R.S. Sheridan Journal of the American Chemical Society 2011, 133, 19688 (Paywall)
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry (where interactive models can be found) with permission
Can a remote substituent affect the singlet-triplet spin state of a carbene? Somewhat surprisingly, the answer is yes. Sheridan has synthesized and characterized the meta and para methoxy-substituted phenyltrifluoromethyl)carbenes 1 and 2. The UV-Vis spectrum of 1 is consistent with a triplet as its EPR and reactivity with oxygen. However, the para isomer 2 gave no EPR signal and failed to react with oxygen or hydrogen, suggestive of a singlet.
The triplet of 1 is estimated to be about 4 kcal mol-1 below that of the singlet – larger than the general overestimation of the stability of triplets that beleaguer B3LYP. For 2, B3LYP predicts a singlet ground state.
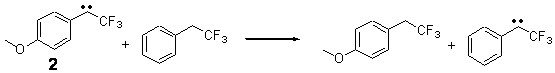
The isodesmic reactions 1 and 2 help understand the strong substituent effect. For 1, the meta substituent destabilizes both the singlet and triplet by a small amount. For 2, the para methoxy group stabilizes the triplet slightly, but stabilizes the singlet by a large amount. This stabilization is likely the result of the contribution of a second resonance structure 2b. A large rotational barrier for both the methyl methyl and the trifluoromethyl groups supports the participation of 2b.

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry (where interactive models can be found) with permission
Can a remote substituent affect the singlet-triplet spin state of a carbene? Somewhat surprisingly, the answer is yes. Sheridan has synthesized and characterized the meta and para methoxy-substituted phenyltrifluoromethyl)carbenes 1 and 2. The UV-Vis spectrum of 1 is consistent with a triplet as its EPR and reactivity with oxygen. However, the para isomer 2 gave no EPR signal and failed to react with oxygen or hydrogen, suggestive of a singlet.
The conformations of 1 and 2 were optimized at B3LYP/6-31+G(d,p) and the lowest energy singlet and triplet conformers are shown in Figure 1. The experimental spectral features of 1 match up best with the computed features of the triplet, and the same is true for the singlet of 2.
The isodesmic reactions 1 and 2 help understand the strong substituent effect. For 1, the meta substituent destabilizes both the singlet and triplet by a small amount. For 2, the para methoxy group stabilizes the triplet slightly, but stabilizes the singlet by a large amount. This stabilization is likely the result of the contribution of a second resonance structure 2b. A large rotational barrier for both the methyl methyl and the trifluoromethyl groups supports the participation of 2b.
ΔEsinglet = -0.8 kcal mol-1
ΔEtriplet = -0.6 kcal mol-1
ΔEsinglet = -5.8 kcal mol-1
ΔEtriplet = -1.1 kcal mol-1

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
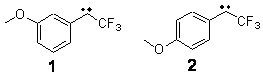
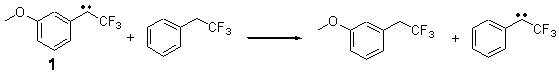
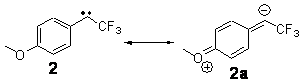
No comments:
Post a Comment